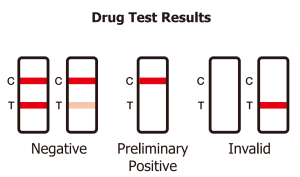
Which drugs does the T-Cube detect and at what concentrations?
T-Cube Oral Fluid – Saliva Drug Test Panel Configurations:
5 Panel
5CO 5 Panel : AMP, COC, MET, OPI, PCP (Does Not Test THC)
6 Panel
6ME 6 Panel : AMP, COC, MET, OPI, PCP, THC (Replace Product Code T-Cube-6MB)
6BPB 6 Panel : AMP, BAR, COC, OPI, PCP, THC (Replaces Product Code T-Cube-6BP)
7 Panel
7PCPB 7 Panel : AMP, COC, MET, OPI, OXY, PCP, THC (Replaces Product Code T-Cube-7PCP and T-Cube-60)
9 Panel
9DBA Panel : AMP, BAR, COC, MDMA, MET, MTD, OPI, OXY, PCP (Does Not Test THC)
10 Panel
10BMP: 10 Panel : AMP, BAR, COC, MDMA, MET, MTD, OPI, OXY, PCP, THC (Replaces Product Code T-Cube-10MO, T-Cube-10BUP, and T-Cube-10PCP)
T-Cube Oral Drug Test Cutoff Levels
| Test | Calibrator | Cutoff Level |
|---|---|---|
| Amphetamine (AMP) | D-Amphetamine | 50 ng/ml |
| Barbiturates (BAR) | Secobarbital | 20 ng/ml |
| Cocaine (COC) | Cocaine | 20 ng/ml |
| Marijuana (THC) | Δ9-THC | 40 ng/ml |
| Methadone (MTD) | Methadone | 30 ng/ml |
| Methamphetamine (MET) | D-Methamphetamine | 50 ng/ml |
| Methylenedioxymethamphetamine (MDMA) | 3,4-Methylenedioxymethamphetamine HCI (MDMA) | 50 ng/ml |
| Opiate (OPI) | Morphine | 40 ng/ml |
| Oxycodone (OXY) | Oxycodone | 20 ng/ml |
| Phencyclidine (PCP) | Phencyclidine | 10 ng/ml |
Please refer to the Package Insert for information about drug tests and cutoffs. Refer to
the ANALYTICAL SENSITIVITY section in the Package Insert for additional details.