When do I read the T-Cube test results?
Read the drug test results within 5 minutes. Do not interpret results after 5 minutes. Results after more than 5 minutes may not be accurate and should not be read.
T-Cube Oral Drug Test FAQ’s. Answers to questions about drug test. Learn how to use the drug test and read the test results.
Frequently asked questions about drug test answered here.
Read the drug test results within 5 minutes. Do not interpret results after 5 minutes. Results after more than 5 minutes may not be accurate and should not be read.
Please refer to the CROSS REACTIVITY section in the Package Insert to determine which substances have been tested and confirmed not to cross react with the specific test.
The cutoff threshold of a drug test is the point of measurement at which a result is considered preliminary positive or negative. If the point of measure is AT or ABOVE the result is considered preliminary positive. If the point of measure is BELOW the result is considered negative. This level is established on the reliability and accuracy of the test and its ability to detect a drug or metabolite for a reasonable period after drug use.
When sufficient oral fluid has been collected, the saturation indicator on the collector will turn red. This should take approximately 3 minutes. If the red color does not appear within 7 minutes, repeat the collection using a new device.
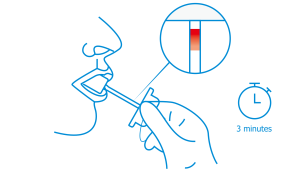
Instruct the donor not to place anything in their mouth. This includes food, drink, gum, and tobacco products. Wait at least 10 minutes for the donors mouth to clear before proceding with collection. No other collection devices should be used with this assay.
Shelf life of T-Cube is up to 24 months when stored at 40-86ºF (4-30ºC).
T-Cube should be stored between 40-86ºF (4-30ºC) in the sealed pouch up to the expiration date. Do not freeze, and keep away from direct sunlight, moisture and heat. Ideally, the test should only be opened shortly before specimen collection and testing.

On July 11th 2017, the Food and Drug Administration (FDA or Agency) announced a list of class II devices that they determined, based on established factors, no longer require premarket notification to provide reasonable assurance of safety and effectiveness, subject to certain limitations.
FDA exemption is limited to test systems intended for Employment and Insurance testing, that include a statement in their labeling saying the device is intended solely for use in Employment and Insurance testing, and does not include test systems intended for Federal drug testing programs (e.g., programs run by the Substance Abuse and Mental Health Services Administration (SAMHSA), the Department of Transportation (DOT), and the U.S. military).
Please refer to the Package Insert/Inner Carton Label to determine whether the T-Cube can be used in Employment and Insurance Testing.
The T-Cube is lateral flow immunochromatographic assay for the qualitative detection of prescription or illegal drugs present in oral fluid specimens. The T-Cube provides only preliminary test results. A more specific alternative analytical method should be used to obtain a confirmed result. Gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-tandem mass spectrometry (LC-MS/MS) are preferred confirmatory methods. Professional judgment should be exercised with any drug test result, particularly when the preliminary result is positive.